views
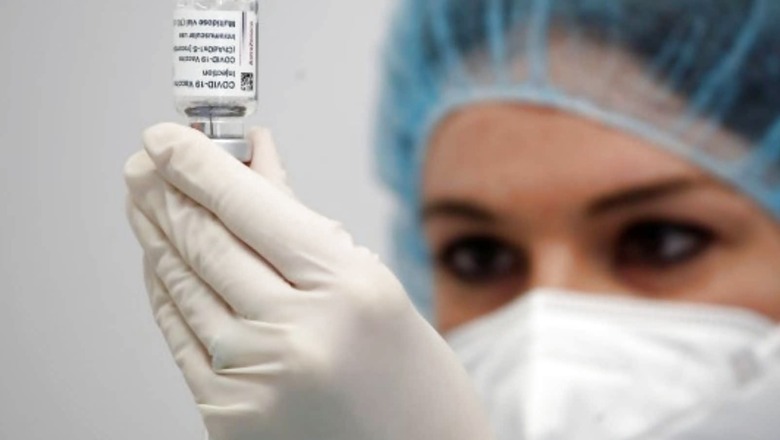
After India’s first indigenous vaccine, Coxavin, received a nod from the World Health Organisation, the Ministry of external affairs (MEA) has reached out to a number of countries to secure approval for the vaccine. As the MEA is holding discussions with counterparts from various countries over Covaxin approval, India’s diplomatic missions, too, are talking with their respective host nations to get their acceptance.
According to a Hindustan Times report, currently, several countries allow fully vaccinated Indian nationals to enter without mandatory quarantine upon arrival. However, most of these countries require travellers to be vaccinated with Covishield, the Indian variant of Britain’s AstraZeneca jab. Covaxin, on the other hand, has received approval from almost a dozen nations. From November 8, the United States, too, will allow entry to travellers who have been vaccinated with Covaxin.
Bharat Biotech on Wednesday said the emergency use listing (EUL) granted by the World Health Organisation to its COVID-19 vaccine Covaxin is a significant step towards ensuring wider global access to the indigenously developed jab.
In a tweet, the WHO said it has granted emergency use listing (EUL) to Covaxin, adding to a growing portfolio of vaccines validated by the global health body for the prevention of COVID-19.
Covaxin is a whole virion-inactivated vaccine against SARS-CoV2, developed in partnership with ICMR and NIV, Pune, Bharat Biotech said in a statement. With validation from the World Health Organisation (WHO), countries can now expedite their regulatory approval processes to import and administer Covaxin, it added.
United Nations Children’s Fund (UNICEF), Pan-American Health Organisation (PAHO) and GAVI COVAX facility will also be able to procure Covaxin for distribution to countries worldwide, Bharat Biotech said.
“Validation by WHO is a very significant step towards ensuring global access to India’s widely administered, safe, and efficacious Covaxin,” Bharat Biotech Chairman and Managing Director Krishna Ella said.
Read all the Latest India News here

















Comments
0 comment