views
Two Chinese pharma companies, which supplied 5.5 lakh rapid testing kits for COVID-19 to India, said they are ready to cooperate with Indian agencies looking into allegations of poor accuracy of their products.
In separate statements, Guongzhou Wondfo Biotech and Livzon Diagnostics said they follow strict quality control of their products, asserting that specified guidelines must be followed in storage of the kits as as well in their usages to get accurate results.
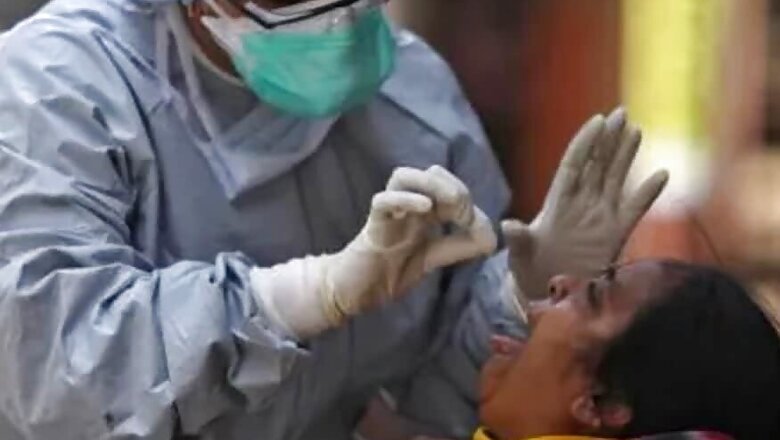
India's apex medical research body ICMR on Tuesday advised states to stop using the rapid antibody test kits for next two days till it examines their quality following complaints that they are not fully effective in detecting coronavirus infection.
Last week, India procured 5.5 lakh rapid antibody test kits from these two Chinese firms and they were distributed to several states reporting rising cases of coronavirus infection.
While Guongzhou Wondfo Biotech supplied 3 lakh test kits, Livzon Diagnostics delivered 2.5 lakh.
Livzon Diagnostics, in its statement, said, "We are shocked to receive negative reports from India related to poor accuracy of COVID 19 rapid testing kits made in China including our brand. We have shown great concern on this issue, and we are willing to coordinate with the related government departments for investigation."
The company said it fulfilled all required quality control standards prescribed by the Chinese government and that the company has been exporting the product to at least 10 countries, including Brazil, Peru, Columbia and several European nations.
"The test kits should be stored at a temperature between 2 and 30 degree celsius and should not be frozen... If the storage temperature is too high, the accuracy of the test may be influenced," it said.
Guongzhou Wondfo Biotech said the company has been exporting the kits to more than 70 countries and that the product was validated and approved by the ICMR through National Institute of Virology in Pune.
"In the process of exporting the products to overseas countries, Wondfo fully cooperates with relevant health authorities in each country to carry out various verifications on the sensitivity and specificity of our products," it said.
The statements by the two companies were released to the media by an official of the Chinese embassy here.
At present, the government hospitals have been using the polymerase chain reaction (PCR) tests to detect coronavirus from throat or nasal swab samples of people. These take around five to six hours to show the results.
In the rapid antibody tests, the blood samples of suspected patients are taken, and it normally takes around 15-30 minutes to give the result. India has been facing severe shortage of testing kits in view of rising cases of the coronavirus.




















Comments
0 comment