
views
Inflating the Balloon
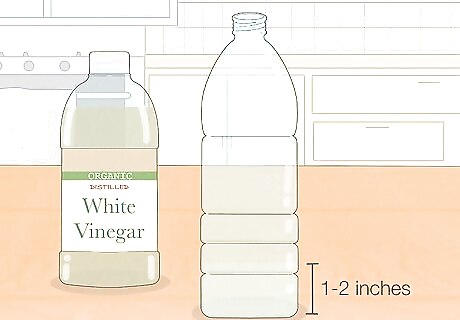
Pour a little vinegar into a plastic bottle. Choose a plastic water bottle, or another bottle with a narrow neck. Pour 1–2 inches (2.5–5 cm) of vinegar into the bottle, using a funnel if you have one. Use white vinegar, also called distilled vinegar, for the best result. You can try this with any kind of vinegar, but the inflation might take longer or require more vinegar to work. Other types of vinegar tend to be more expensive as well. Vinegar can damage metal containers, potentially adding an unpleasant taste to food and drink stored in that container. If you have no plastic bottles, use a high-quality stainless steel bottle to minimize the chance of this happening. Weakening the vinegar with an equal amount of water might also help, and won't prevent the balloon from inflating.
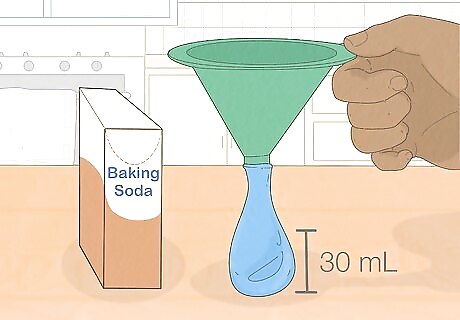
Use a funnel or straw to put a little baking soda into a limp balloon. You can use any shape and color of balloon. Hold it loosely by the neck, with the open side of the balloon facing towards you. Fit a funnel into the neck if you have one, then pour about two tablespoons (30 mL) baking soda into the balloon, or just fill the balloon about halfway full. If you don't have a funnel, you can place a plastic straw into a pile of baking soda, put your finger over the top hole of the straw, then poke the straw into the balloon and lift your finger. Tap the straw to get the baking soda to fall out, and repeat until the balloon is at least 1/3 of the way full.
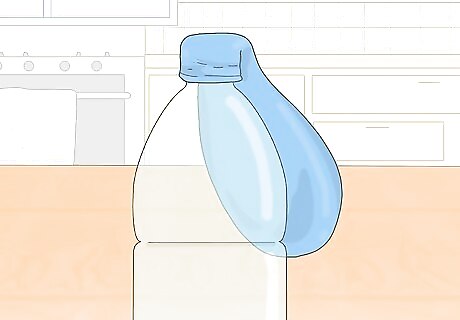
Stretch the neck of the balloon over the top of the bottle. Be careful not to spill the baking soda while you do this. Hold the balloon's neck with both hands and stretch it over the top of the plastic bottle containing vinegar. Have a friend keep the bottle steady if the table or bottle is wobbly.
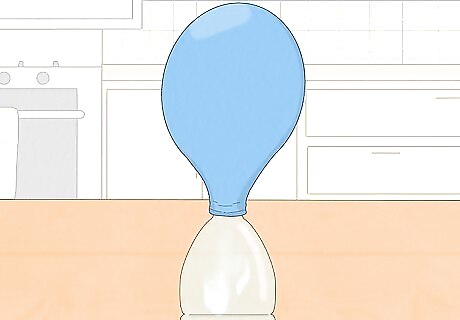
Lift the balloon up over the bottle and watch the reaction. The baking soda should fall out of the balloon, through the neck of the bottle, and into the vinegar at the bottom. Here, the two chemicals will fizz and react, turning into other chemicals. One of these is carbon dioxide, a gas, which will rise up and inflate the balloon. Shake the bottle gently to mix the two ingredients if there's not much fizzing.

Try again with more vinegar or baking soda, if it doesn't work the first time. If the fizzing has stopped and the balloon still hasn't inflated after you count to 100, empty out the bottle and try again with more vinegar and baking soda. The stuff left in the bottle has turned into other chemicals, mostly water, so it can't be used again. Don't go overboard. The bottle should never be more than about 1/3 full of vinegar.
Grasping how the Process Works

Learn about chemical reactions. Just about everything around you is made up of molecules, or different types of substances. Often, two kinds of molecules react with each other, breaking up and forming different molecules out of the pieces.

Learn about baking soda and vinegar. The reactants, or substances that reacted with each other in the fizzy reaction you saw, are baking soda and vinegar. Unlike many ingredients in your kitchen, both of these are simple chemicals, not complicated mixtures of many chemicals: Baking soda is another word for the molecule sodium bicarbonate. White vinegar is a mixture of acetic acid and water. Only the acetic acid reacts with the baking soda.

Read about the reaction. Baking soda is a type of substance called a base. Vinegar, or acetic acid, is a type of substance called an acid. Bases and acids react with each other, partially breaking apart and forming different substances. This is described as "neutralization" because the end result is neither a base nor an acid. In this case, the new substances are water, a kind of salt, and carbon dioxide. Carbon dioxide, a gas, leaves the liquid mixture and expands throughout the bottle and the balloon, inflating it. Although the definition of acid and base can get complicated, you can compare the differences between the original substances and the "neutralized" result to see there are obvious changes. For instance, vinegar has a strong smell and can be used to dissolve grime and dirt. After being mixed with baking soda, it smells much less strongly and is no more effective at cleaning than water is.

Study the chemical formula. If you're familiar with some chemistry, or curious about how scientists describe reactions, the formula below describes the reaction between sodium bicarbonate NaHCO3 and acetic acid HC2H3O2(aq). Can you figure out how each molecule splits apart and reforms? NaHCO3 + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g) The letters in parentheses show the state the chemicals are in during and after the reaction: (g)as, (l)iquid, or (aq)ueous. "Aqueous" means the chemical is dissolved in water.


















Comments
0 comment