views
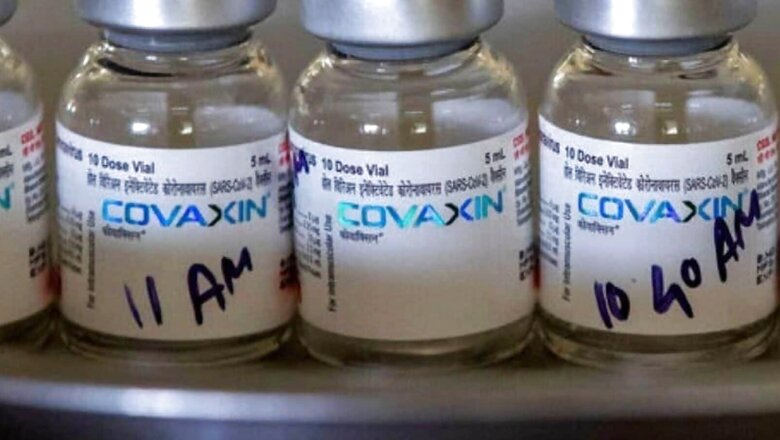
To get Covaxin approved by Canada, Bharat Biotech needs to submit an application with all data and evidence related to the clinical trials, it was reported on Tuesday.
According to Times of India, the Canadian High Commission has said that the vaccine manufacturers “need to submit an application with all data and evidence as to the clinical trials conducted and the scientific data about its safety and efficiency.”
Also Read: These Countries Have Approved Covaxin, But Travel For Indians Remains Distant Dream
“Canada does not review every vaccine the (WHO) has accepted…. the makers of Covaxin have not made a submission to Health Canada for approval of their vaccine. So Bharat Biotech needs to submit their application for Canada to review this vaccine,” a High Commission spokesperson was quoted as saying in the TOI report.
Canada is yet to accept the India made vaccine despite World Health Organization’s approval for emergency use.
According to a spokesperson at the Canadian High Commission, the list of approved vaccines for Canada include Pfizer-BioNTech, Moderna, and Johnson & Johnson.
The timing of completion of Health Canada’s review depends on several factors like additional data, discussion with sponsors and safety information.
Also Read: Despite WHO Nod, Canada Yet to Approve Covaxin for Vaccination
A Canadian HC spokesperson had earlier said that Covid-19 drug and vaccine submissions are reviewed on an expedited timeline, “above the usual performance standards, without compromising safety, efficacy and quality standards, due to the public health need”.
“The timing of the completion of Health Canada’s review depends on many factors, including but not limited to a need for additional data, discussions with sponsor and requirements for updates to safety information,” the spokesperson had said.
The WHO last week said that it has granted approval for Bharat Biotech’s home-grown COVID-19 vaccine for emergency use listing, paving the way for it to be accepted as a valid vaccine in many poor countries.
The WHO tweeted that its technical advisory group had ruled that benefits of the shot, known as Covaxin, significantly outweighed the risks and that it met WHO standards for protection against COVID-19.
The decision had been delayed as the advisory group sought additional clarifications from Bharat Biotech before conducting a final risk-benefit assessment for the vaccine’s global use.
WHO’s Strategic Advisory Group of Experts on Immunization also recommended Covaxin’s use in two doses, with an interval of four weeks, in age groups 18 and above. These recommendations are in line with the company’s guidance.
Read all the Latest India News here

















Comments
0 comment