views
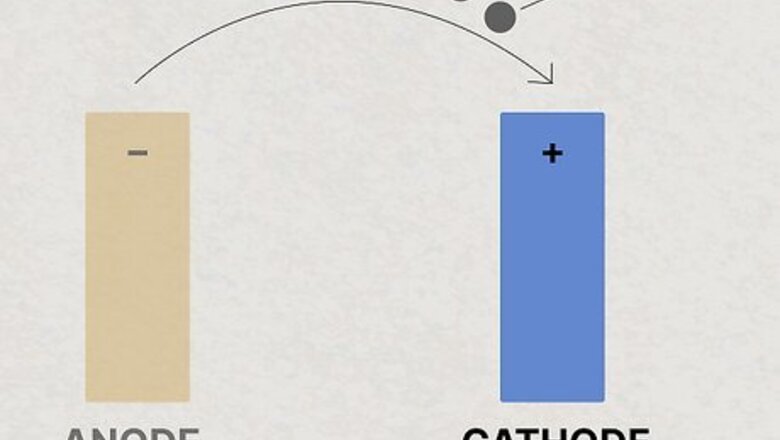
- Cathodes are positive electrodes that receive electrons.
- Anodes are negative electrodes that release electrons.
- Inside of a battery, anodes and cathodes are connected by a metal conductor to pass electrons.
Cathode Definition
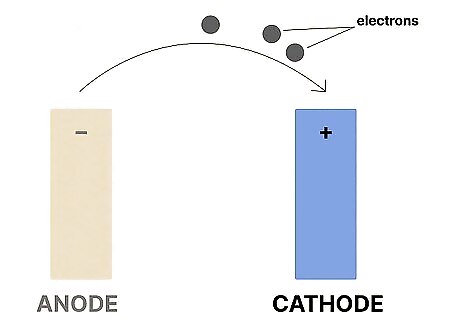
A cathode is the positive electrode that acquires electrons. Also called an oxidizing electron, a cathode acquires electrons from an electrochemical reaction (usually inside of a battery). The cathode receives electrons from the anode.
Cathode Properties
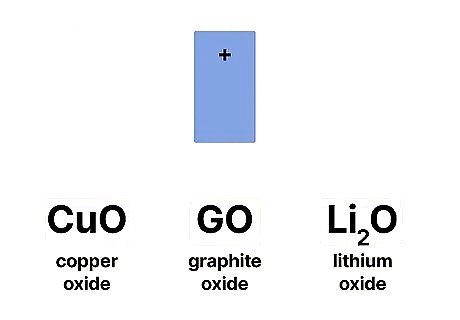
A cathode can be made of any material as long as it’s an oxidizing agent. Typically, this is a metal like copper oxide, graphic oxide, or lithium oxide. In batteries, cathodes have to be stable when in contact with electrolytes, since that’s what aids in the electrochemical reaction.
Anode Definition
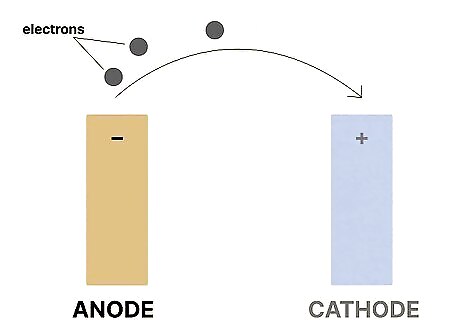
An anode is the negative electrode that releases electrons. Also called the reducing electrode, an anode oxidizes during an electrochemical reaction. As it oxidizes, it releases the electrons that the cathode then acquires. If you’re working on a water heater, you may have heard the term “sacrificial anode.” The sacrificial anode is a metal rod that sits inside of your water heater. It attracts particles (like iron or limestone) and corrodes over time, helping the water heater tank itself avoid corrosion.
Anode Properties
Anodes are made of stable, conductive materials. Typically, they’ll be made of lithium, zinc, graphite, or platinum. Anodes must have good conductivity so that they can release their electrodes and pass them to the cathode.
How Cathodes and Anodes Interact
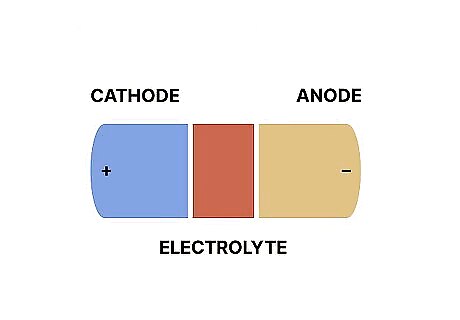
A metal conductor connects cathodes and anodes inside a battery. The conductor is typically a metal wire or metal tube that runs from the cathode to the anode. If the battery has a charge, the anode releases electrons that then run along the conductor and into the cathode. The cathode then acquires those electrons. Once the anode erodes completely (meaning it releases all of its electrons), the battery will die or lose charge. This conductor is also sometimes referred to as an “electrolyte.” While electrolytes usually refer to a liquid conductor, inside of batteries, the electrolyte is a solid conductor.
Cathodes and Anodes in Batteries
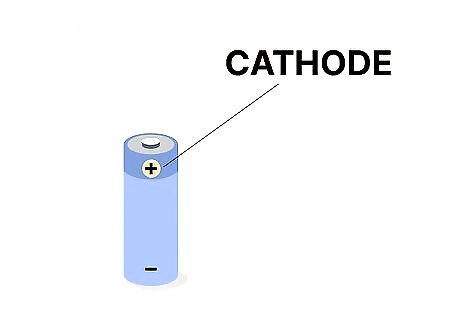
A cathode is indicated by a positive + sign. Take a look at a battery, any kind. You’ll notice a small plus sign, or positive sign, on one side. This is where the cathode is: since it’s a positive electrode, it’s always indicated by the positive sign.
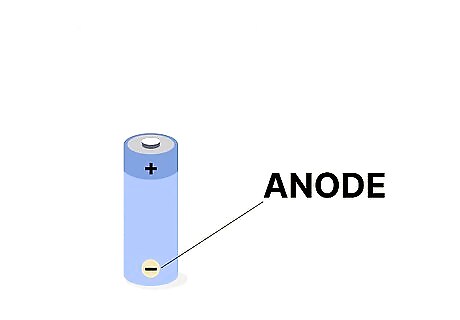
An anode is indicated by a negative - sign. Now, check out the other side of the battery, and look for the small negative, or minus sign. This is the anode. Since it’s a negative electrode, it’s indicated by the negative sign. It’s important to know where cathodes and anodes are, especially if you’re jump-starting a battery (like in a car). Attaching the jumper cables to the cathode and anode connections in the right order is crucial for a clean charge. You can also find cathodes and anodes in other utilities, like water heaters and fluid tanks.














Comments
0 comment