views
Deriving the Molecular Formula from an Empirical Formula
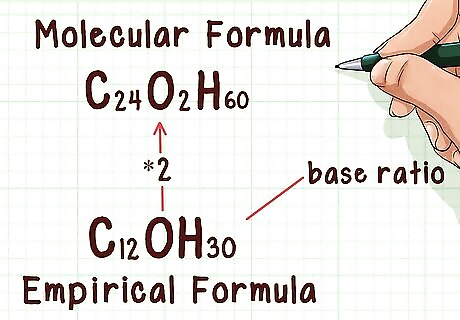
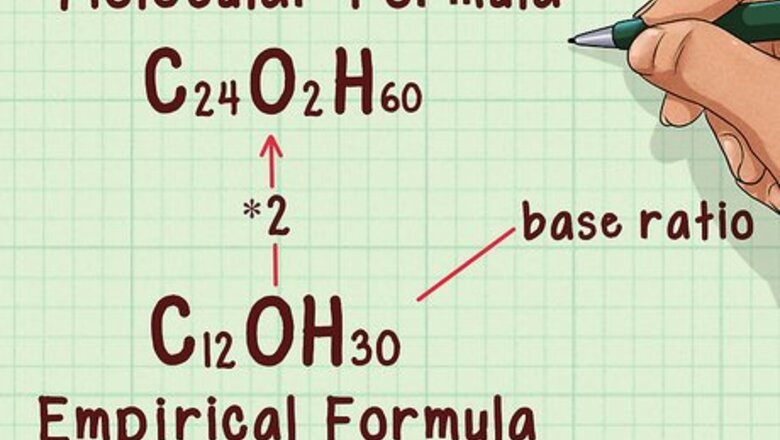
Know the relationship between molecular and empirical formulae. The empirical formula provides the simplest, most reduced ratio of elements within a molecule, for example, two oxygens for every carbon. The molecular formula tells you how many of each of those atoms is present in the molecule. For example, one carbon and two oxygens (carbon dioxide). The two formulae are related by a whole number ratio such that if the empirical formula is multiplied by the ratio, it will yield the molecular formula.
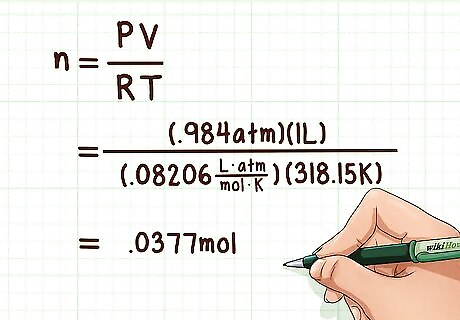
Calculate the number of moles of gas. This means utilizing the ideal gas law. You can determine the number of moles based on the pressure, volume, and temperature provided by the experimental data. The number of moles can be calculated using the following formula: n = PV/RT. In this formula, n is the number of moles, P is the pressure, V is the volume, T is the temperature in Kelvin, and R is the gas constant. Example: n = PV/RT = (0.984 atm * 1 L) / (0.08206 L atm mol-1 K-1 * 318.15 K) = 0.0377 mol
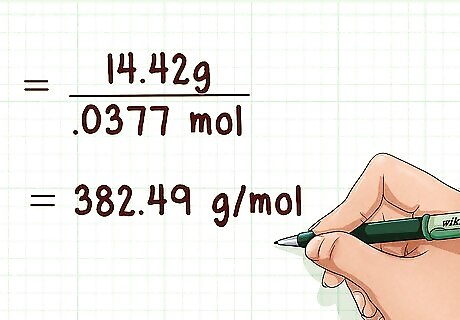
Calculate the molecular weight of the gas. This can only be done after finding the moles of gas present using the ideal gas law. You will also need to know how many grams of gas were present. Then divide the grams of gas by the moles of gas present to yield molecular weight. Example: 14.42 g / 0.0377 mol = 382.49 g/mol
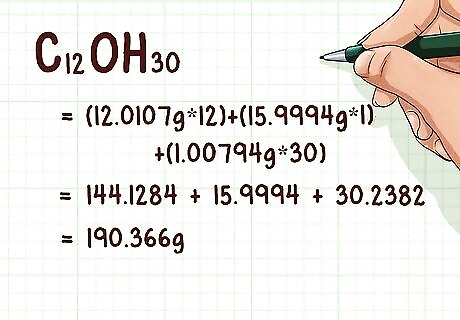
Add together the atomic weight of all atoms in the empirical formula. Each atom in the empirical formula has its own atomic weight. This value can be found at the bottom of the atom’s square on the periodic table. Add these weights together to get the weight of the empirical formula. For example, carbon has an atomic weight of 12.0107, hydrogen has an atomic weight of 1.00794, and oxygen has an atomic weight of 15.9994. It's okay to look up the atomic weight if you don't know it. Example: (12.0107 g * 12) + (15.9994 g * 1) + (1.00794 g * 30) = 144.1284 + 15.9994 + 30.2382 = 190.366 g
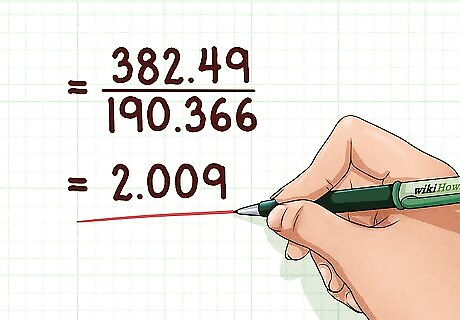
Find the ratio between the molecular and empirical formula weights. In doing this, you can determine how many times the empirical weight is repeated within the actual molecule. Knowing how many times the empirical weight is repeated will let you find the number of times that the empirical formula repeats itself in the molecular formula. This should be a whole number. If the ratio is not a whole number, you will have to round it. Example: 382.49 / 190.366 = 2.009
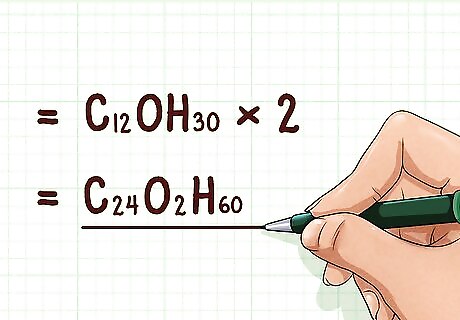
Multiply the empirical formula by the ratio. Multiply the subscripts of the empirical formula by the ratio. This will yield the molecular formula. Note that for any compound with a ratio of “1,” the empirical formula and molecular formula will be the same. Example: C12OH30 * 2 = C24O2H60
Finding the Empirical Formula
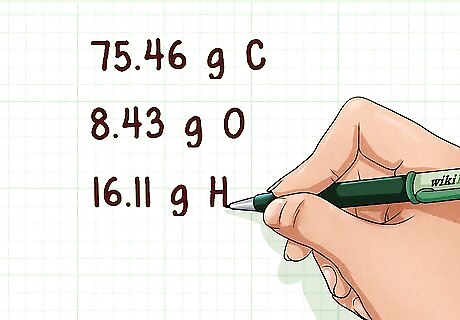
Find the mass of each atom present. Sometimes the mass of each atom will be given. Other times, it will be given as a percent mass. If this is the case, assume a 100 g sample of the compound. This will allow you to write the percent mass as an actual mass in grams. Example: 75.46 g C, 8.43 g O, 16.11 g H
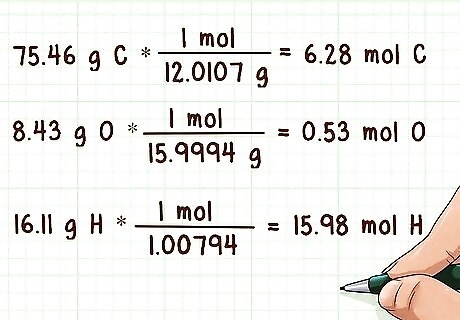
Convert the masses to moles. You need to convert the molecular masses of each element to moles. In order to do this, you need to divide the molecular masses by the atomic masses of each respective element. You can find the atomic mass in the bottom of that element’s square on the periodic table. Example: 75.46 g C * (1 mol / 12.0107 g) = 6.28 mol C 8.43 g O * (1 mol / 15.9994 g) = 0.53 mol O 16.11 g H * (1 mol / 1.00794) = 15.98 mol H
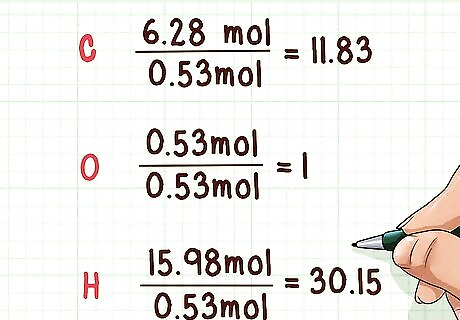
Divide all molar values by the smallest molar value. You need to divide the number of moles for each separate element by the smallest molar amount from all the elements present in the compound. In doing so, you can find the simplest mole ratios. This works, because it gives sets the least abundant element to “1” and provides the respective ratios of other elements in the compound. Example: Smallest molar amount is oxygen with 0.53 mol. 6.28 mol/0.53 mol = 11.83 0.53 mol/0.53 mol = 1 15.98 mol/0.53 mol= 30.15
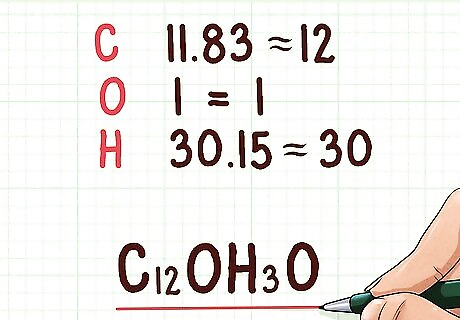
Round your molar values to whole numbers. These numbers will become the subscripts in the empirical formula. You should round them to the nearest whole number. After finding these numbers, you can write the empirical formula. Example: The empirical formula would be C12OH30 11.83 = 12 1 = 1 30.15 = 30
Understanding Chemical Formulae
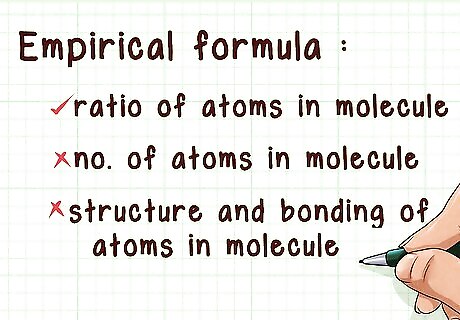
Understand an empirical formula. An empirical formula gives you information about the molar ratios of one atom to another in a molecule. This does not provide any information about exactly how many atoms are present in the molecule. The empirical formula also fails to provide information about the structure and bonding of atoms in a molecule.
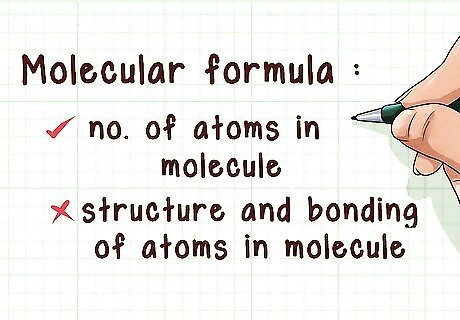
Know what a molecular formula tells you. Like the empirical formula, the molecular formula fails to provide information about the bonding and structure of a molecule. Unlike the empirical formula, the molecular formula gives you details about how many of each atom is present in the molecule. The empirical formula and molecular formula are related by a whole number ratio.
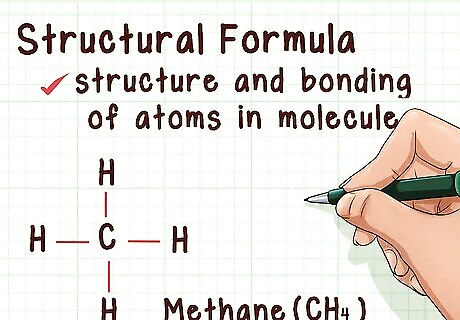
Understand structural representations. Structural representations give even more information than molecular formulas. In addition to showing how many of each atom is present in a molecule, structural representations give you information about the bonding and structure of the molecule. This information is crucial to understanding the way the molecule will react. There are several different types of structural representations, which show you different things about the compound. For example, it might show the compound's connectivity or its molecular shape, such as by drawing dashed lines to indicate their bonds.


















Comments
0 comment