views
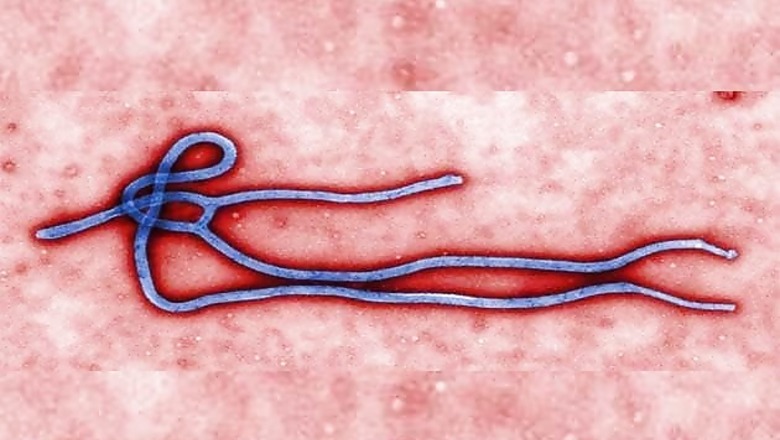
Geneva: The World Health Organization voiced hope that the number of Ebola cases would start falling sharply early next year, stressing the need to develop rapid diagnostic tests for the end-phase.
"We are looking at where we will be in four to six months from now when the cases (could) decrease sharply and we will try to find the very last cases," Pierre Formenty, a top WHO Ebola expert, told reporters in Geneva.
The UN health agency thinks that with continued intense international efforts, the outbreak which has killed nearly 5,200 people, almost all of them in West Africa, could begin slowing during the first months of 2015.
"We're not saying it's over," Formenty stressed, but he said the hope was that by March the three countries ravaged by the deadly virus Guinea, Liberia and Sierra Leone would each be seeing only between five and 10 cases a week.
When the outbreak does slow down, there will be a need to carry out far more diagnostic test in far more settings, "to make sure that we are not missing cases," he said.
The problem is that the tests that exist today are "cumbersome, slow and complex", WHO acknowledged, pointing out that they required "high-level laboratory biosafety and staff expertise in using sophisticated machines."
The standard tests include the so-called reverse-transcriptase polymerase chain reaction, or RT-PCR test, involving laborious procedures, and require a full tube of blood.
It trained staff between two to six hours to get results, and each test costs around $100 a piece.
Even with the number of fully-equipped mobile labs in the three countries set to be scaled up from 12 today to between 17 and 20 by the end of the year, "these requirements are difficult to meet," WHO said.
The agency is therefore calling for companies to develop far faster and nimbler tests, that can be conducted in regular health care facilities to check every patient who comes in with fever for the deadly virus.
WHO has drawn up a wish-list of criteria for interested companies to draw from.
The test should be suited for use in regular health care clinics without laboratories, should involve fewer than three steps and produce results in less than 30 minutes.
And of course, health personnel must be able to carry them out safely, and they should be easy to store, and inexpensive, WHO said.
Fifteen companies have already proposed 17 different products, including tests that resemble standard pregnancy tests in their simplicity, WHO said.
The agency will host a special meeting to discuss the issue on December 12.



















Comments
0 comment