views
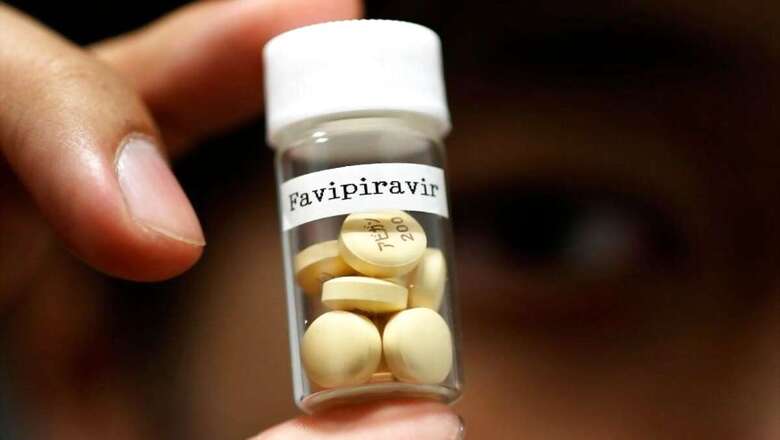
Zenara Pharma, a fully owned subsidiary of Biophore India Pharmaceuticals, announced that it has received approval from the Drugs Controller General of India (DCGI), to manufacture and sell Favipiravir tablets as a treatment option for patients with mild to moderate symptoms of COVID-19.
The tablet, which will be sold under the brand name 'Favizen', is being manufactured at Zenar's US FDA approved facility here, a press release from the drug maker said.
Jagadeesh Babu Rangisetty, Co-founder and Managing Director of the firm, said, "In the ongoing pandemic, it has become extremely critical for pharmaceutical companies to quickly provide safe and effective treatment options for patients with COVID-19. I am proud to say that we have our own in-house API and are not dependent on any imports for the production. We believe that this will ensure stability and rapid production and availability of this treatment for the Indian market."
The company is also in talks with various institutions to make the tablets available at discounted or no cost to underprivileged patients, he added.
Zenara Pharma is in talks with multiple state institutions and several hospitals in India to ensure that Favizen is readily available for patients in need.
The company is also likely to tie up with other partners across the country to increase patient coverage, it said.
Internationally, Zenara has the manufacturing and distribution capabilities to improve access to this treatment around the world and has already begun exports to the Middle East and Latin American countries.




















Comments
0 comment