
views
Review
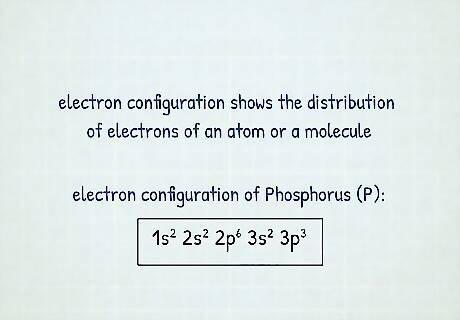
What is an electron configuration? An electron configuration shows the distribution of electrons of an atom or a molecule. There is a specific notation that can quickly show you where the electrons are likely to be located, so knowing this notation is an essential part of knowing electron configurations. Reading these notations can tell you what element you’re referring to and how many electrons it has. The structure of the periodic table is based on electron configuration. For example, the notation for Phosphorus (P) is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}}.
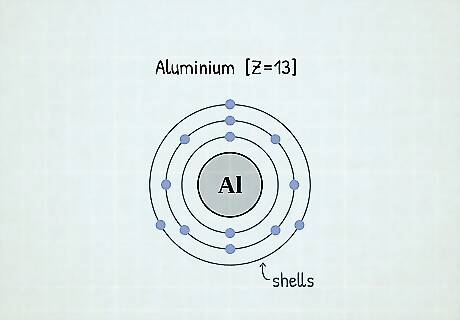
What are electron shells? The area that surrounds the nucleus of an atom, or the area where the electrons orbit, is called an electron shell. There are usually around 3 electron shells per atom, and the arrangement of these shells is called the electron configuration. All electrons in the same shell must have the same energy. Electron shells are also sometimes referred to as energy levels.
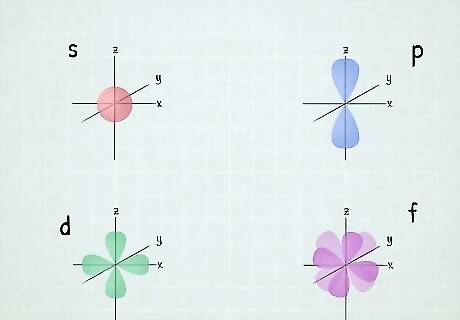
What is an atomic orbital? As an atom gains electrons, they fill different orbitals sets according to a specific order. Each set of orbitals, when full, contains an even number of electrons. The orbital sets are: The s orbital set (any number in the electron configuration followed by an "s") contains a single orbital, and by Pauli's Exclusion Principle, a single orbital can hold a maximum of 2 electrons, so each s orbital set can hold 2 electrons. The p orbital set contains 3 orbitals, and thus can hold a total of 6 electrons. The d orbital set contains 5 orbitals, so it can hold 10 electrons. The f orbital set contains 7 orbitals, so it can hold 14 electrons. The g, h, i and k orbital sets are theoretical. No known atoms have electrons in any of these orbitals. The g set has 9 orbitals, so it could theoretically contain 18 electrons. The h set would have 11 orbitals and a maximum of 22 electrons, the i set would have 13 orbitals and a maximum of 26 electrons, and the k set would have 15 orbitals and a maximum of 30 electrons. Remember the order of the letters with this mnemonic: Sober Physicists Don't Find Giraffes Hiding In Kitchens.
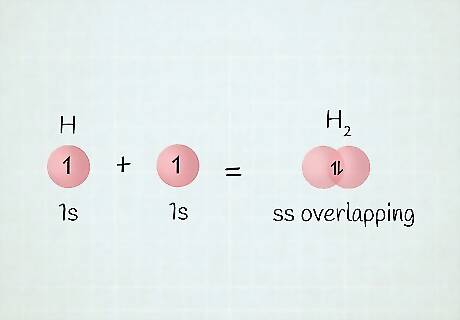
What are overlap orbitals? Sometimes, electrons occupy a shared orbital space. Take the Dihydrogen molecule, or H2. The 2 electrons must stay close to each other in order to stay attracted to each other and connect. Since they’re so close, they will occupy the same orbital space, thus sharing the orbital, or overlapping it. In your notation, you’d simply change the row number to 1 less than it actually is. For example, the electron configuration for germanium (Ge) is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 . {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{10}4p^{2}.} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{10}4p^{2}.} Even though you go all the way to row 4, there is still a “3d” in the middle there because of overlap.
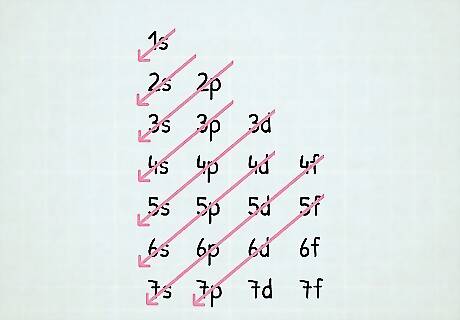
How do you use an electron configuration table? If you’re having trouble visualising your notation, it can be useful to use an electron configuration table so you can actually see what you’re writing. Set up a basic table with the energy levels going down the y-axis and the orbital type going across the x-axis. From there, you can draw your notation in the corresponding spaces as they go down the y-axis and across the x-axis. Then, you can follow the line to get your notation. For example, if you were writing out the configuration for beryllium, you’d start up at the 1s, then loop back around to the 2s. Since beryllium only has 4 electrons, you’d stop after that, and get your notion of 1 s 2 2 s 2 . {\displaystyle 1s^{2}2s^{2}.} {\displaystyle 1s^{2}2s^{2}.}
Assigning Electrons Using a Periodic Table
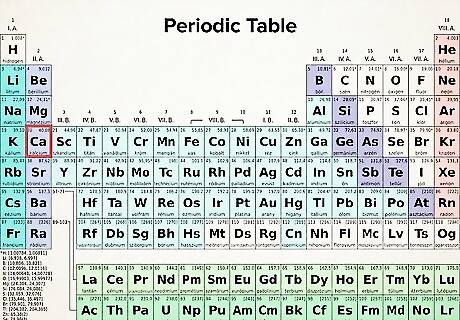
Find your atom's atomic number. Each atom has a specific number of electrons associated with it. Locate your atom's chemical symbol on the periodic table. The atomic number is a positive integer beginning at 1 (for hydrogen) and increasing by 1 for each subsequent atom. The atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Since the periodic table is based on electron configuration, you can use it to determine the element’s configuration notation.
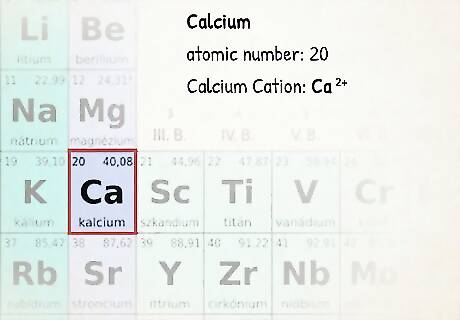
Determine the charge of the atom. Uncharged atoms will have exactly the number of electrons as is represented on the periodic table. However, charged atoms (ions) will have a higher or lower number of electrons based on the magnitude of their charge. If you're working with a charged atom, add or subtract electrons accordingly: add 1 electron for each negative charge and subtract 1 for each positive charge. For instance, a sodium atom with a +1 charge would have an electron taken away from its basic atomic number of 11. So, the sodium atom would have 10 electrons in total. A sodium atom with a -1 charge would have 1 electron added to its basic atomic number of 11. The sodium atom would then have a total of 12 electrons.
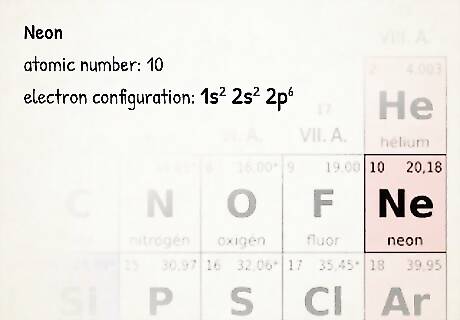
Understand electron configuration notation. Electron configurations are written so as to clearly display the number of electrons in the atom as well as the number of electrons in each orbital. Each orbital is written in sequence, with the number of electrons in each orbital written in superscript to the right of the orbital name. The final electron configuration is a single string of orbital names and superscripts. For example, here is a simple electron configuration: 1s 2s 2p. This configuration shows that there are 2 electrons in the 1s orbital set, 2 electrons in the 2s orbital set, and 6 electrons in the 2p orbital set. 2 + 2 + 6 = 10 electrons total. This electron configuration is for an uncharged neon atom (neon's atomic number is 10.)
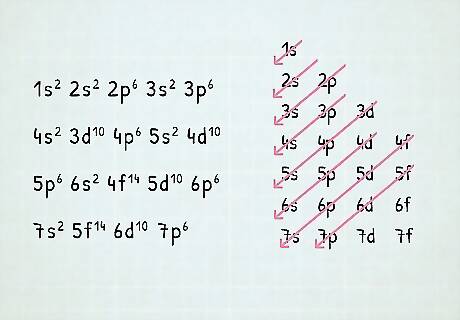
Memorize the order of the orbitals. Note that orbital sets are numbered by electron shell, but ordered in terms of energy. For instance, a filled 4s is lower energy (or less potentially volatile) than a partially-filled or filled 3d, so the 4s shell is listed first. Once you know the order of orbitals, you can simply fill them according to the number of electrons in the atom. The order for filling orbitals is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s. An electron configuration for an atom with every orbital completely filled would be written: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d7p Note that the above list, if all the shells were filled, would be the electron configuration for Og (Oganesson), 118, the highest-numbered atom on the periodic table—so this electron configuration contains every currently known electron shell for a neutrally charged atom.
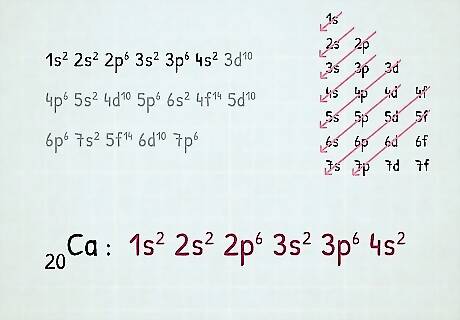
Fill in the orbitals according to the number of electrons in your atom. For instance, if we want to write an electron configuration for an uncharged calcium atom, we'll begin by finding its atomic number on the periodic table. Its atomic number is 20, so we'll write a configuration for an atom with 20 electrons according to the order above. Fill up orbitals according to the order above until you reach 20 total electrons. The 1s orbital gets 2 electrons, the 2s gets 2, the 2p gets 6, the 3s gets 2, the 3p gets 6, and the 4s gets 2 (2 + 2 + 6 +2 +6 + 2 = 20.) Thus, the electron configuration for calcium is: 1s 2s 2p 3s 3p 4s. Note: Energy level changes as you go up. For example, when you are about to go up to the 4th energy level, it becomes 4s first, then 3d. After the 4th energy level, you'll move onto the 5th where it follows the order once again (5s, then 4d). This only happens after the 3rd energy level.
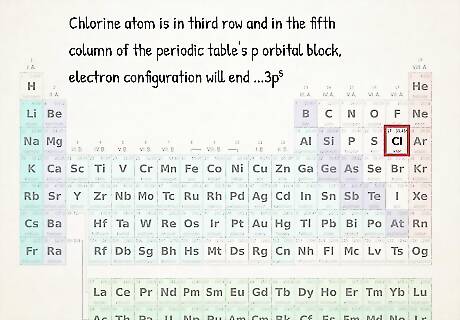
Use the periodic table as a visual shortcut. You may have already noticed that the shape of the periodic table corresponds to the order of orbital sets in electron configurations. For example, atoms in the second column from the left always end in "s", atoms at the far right of the skinny middle portion always end in "d," etc. Use the periodic table as a visual guide to write configurations – the order that you add electrons to orbitals corresponds to your position in the table. Specifically, the 2 leftmost columns represent atoms whose electron configurations end in s orbitals, the right block of the table represents atoms whose configurations end in p orbitals, the middle portion, atoms that end in d orbital, and the bottom portion, atoms that end in f orbitals. For example, when writing an electron configuration for Chlorine, think: "This atom is in third row (or "period") of the periodic table. It's also in the fifth column of the periodic table's p orbital block. Thus, its electron configuration will end ...3p Caution: the d and f orbital regions of the table correspond to energy levels that are different from the period they're located in. For instance, the first row of the d orbital block corresponds to the 3d orbital even though it's in period 4, while the first row of the f orbital corresponds to the 4f orbital even though it's in period 6.
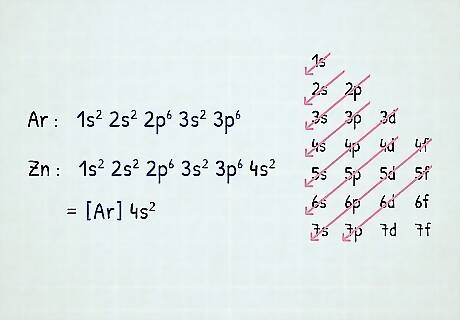
Learn shorthand for writing long electron configurations. The atoms along the right edge of the periodic table are called noble gases. These elements are very chemically stable. To shorten the process of writing a long electron configuration, simply write the chemical symbol of the nearest chemical gas with fewer electrons than your atom in brackets, then continue with the electron configuration for the following orbital sets. To understand this concept, it's useful to write an example configuration. Let's write a configuration for zinc (atomic number 30) using noble gas shorthand. Zinc's full electron configuration is: 1s 2s 2p 3s 3p 4s 3d. However, notice that 1s 2s 2p 3s 3p is the configuration for Argon, a noble gas. Just replace this portion of zinc's electron notation with Argon's chemical symbol in brackets ([Ar].) So, zinc's electron configuration written in shorthand is [Ar]4s 3d. Note that if you are doing noble gas notation for, say, argon, you cannot write [Ar]! You have to use the noble gas that comes before that element; for argon, that would be neon ([Ne]).
Using an ADOMAH Periodic Table
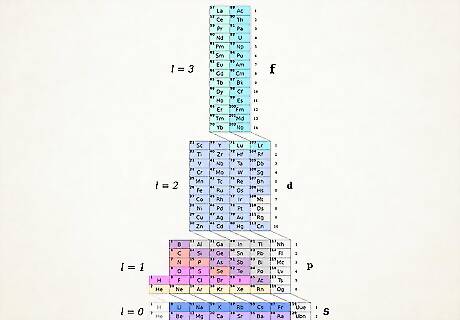
Understand the ADOMAH Periodic Table. This method of writing electron configurations doesn't require memorization. However, it does require a rearranged periodic table, because in a traditional periodic table, beginning with 4th row, period numbers do not correspond to the electron shells. Find an ADOMAH Periodic Table, a special type of periodic table designed by scientist Valery Tsimmerman. It's easily found via a quick online search. In the ADOMAH Periodic Table, horizontal rows represent groups of elements, such as halogens, inert gases, alkali metals, alkaline earths, etc. Vertical columns correspond to electron shells and so called “cascades” (diagonal lines connecting s,p,d and f blocks) correspond to periods. Helium is moved next to Hydrogen, since both of them are characterized by the 1s orbital. Blocks of periods (s,p,d and f) are shown on the right side and shell numbers are shown at the base. Elements are presented in rectangular boxes that are numbered from 1 to 120. These numbers are normal atomic numbers that represent total number of electrons in a neutral atom.
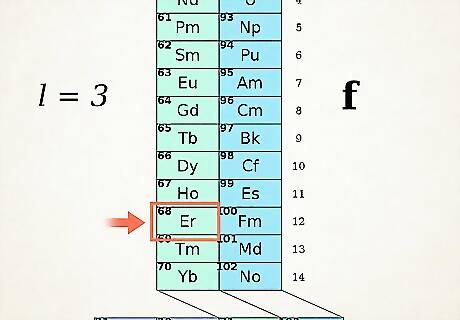
Find your atom in the ADOMAH table. To write electron configuration of an element, locate its symbol in ADOMAH Periodic Table and cross out all elements that have higher atomic numbers. For example, if you need to write electron configuration of Erbium (68), cross out elements 69 through 120. Notice numbers 1 through 8 at the base of the table. These are electron shell numbers, or column numbers. Ignore columns which contain only crossed out elements. For Erbium, remaining columns are 1,2,3,4,5 and 6.
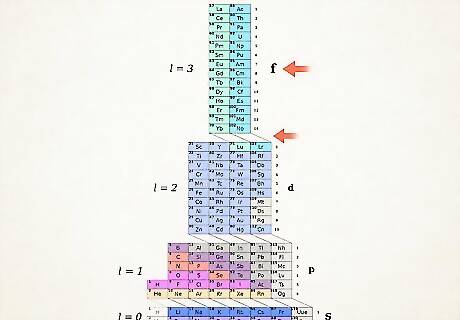
Count orbital sets up to your atom. Looking at the block symbols shown on the right side of the table (s, p, d, and f) and at the column numbers shown at the base and ignoring diagonal lines between the blocks, break up columns into column-blocks and list them in order from the bottom up. Again, ignore column blocks where all elements are crossed out. Write down the column-blocks beginning with the column number followed by the block symbol, like this: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 6s (in case of Erbium). Note: The above electron configuration of Er is written in the order of ascending shell numbers. It could also be written in the order of orbital filling. Just follow cascades from top to bottom instead of columns when you write down the column-blocks: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f.
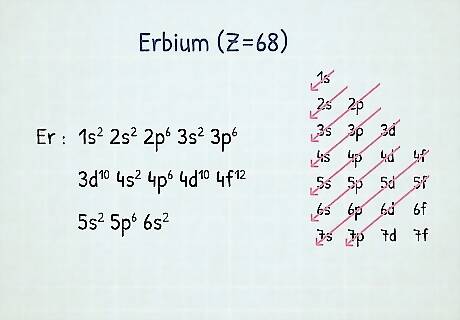
Count electrons for each orbital set. Count elements that were not crossed out in each block-column, assigning 1 electron per element, and write down their quantity next to the block symbols for each block-column, like this: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 6s. In our example, this is the electron configuration of Erbium.
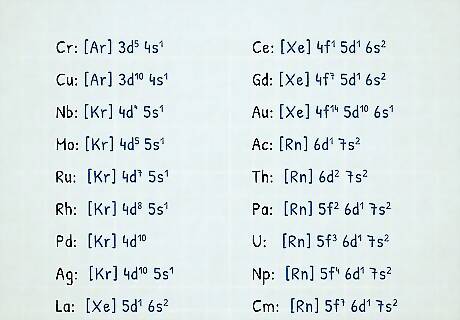
Know irregular electron configurations. There are eighteen common exceptions to electron configurations for atoms in the lowest energy state, also called the ground state. They deviate from the general rule only by last 2 to 3 electron positions. In these cases, the actual electron configuration keeps the electrons in a lower-energy state than in a standard configuration for the atom. The irregular atoms are: Cr (..., 3d5, 4s1); Cu (..., 3d10, 4s1); Nb (..., 4d4, 5s1); Mo (..., 4d5, 5s1); Ru (..., 4d7, 5s1); Rh (..., 4d8, 5s1); Pd (..., 4d10, 5s0); Ag (..., 4d10, 5s1); La (..., 5d1, 6s2); Ce (..., 4f1, 5d1, 6s2); Gd (..., 4f7, 5d1, 6s2); Au (..., 5d10, 6s1); Ac (..., 6d1, 7s2); Th (..., 6d2, 7s2); Pa (..., 5f2, 6d1, 7s2); U (..., 5f3, 6d1, 7s2); Np (..., 5f4, 6d1, 7s2) and Cm (..., 5f7, 6d1, 7s2).
Special Cases and Exceptions
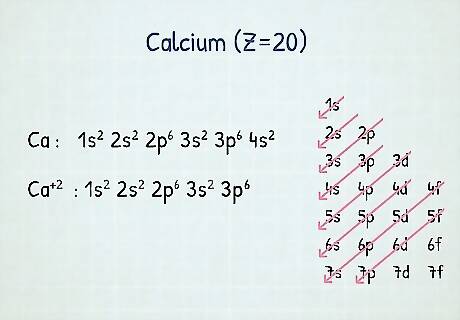
Notating cations: When you’re dealing with cations, it’s very similar to neutral atoms in a grounded state. Start by removing electrons in the outermost p orbital, then the s orbital, then the d orbital. For example, ground state electronic configuration of calcium (Z=20) is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}}. The calcium ion, however, has 2 less electrons, so you’d start by removing them from the outermost shell (which is 4). So, the configuration for calcium ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}}.
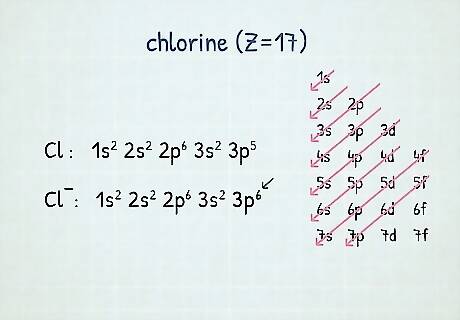
Notating anions: When you notate an anion, you have to use the Aufbau Principle, which states that electrons fill the lowest available energy levels first before moving onto higher ones. So, you’d add electrons to the outermost energy level (or the lowest), before moving inwards to add more. For example, neutral chlorine (Z=17) has 17 electrons and is notated as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5 {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{5}} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{5}}. The chloride ion, however, has 18 electrons, which you’d add starting at the outermost energy level. Therefore, the chloride ion is notated as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}} {\displaystyle 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}}.

Chromium and Copper: As with every rule, there are exceptions. Although most elements follow the Aufbau Principle, these elements do not. Instead of going to the lowest energy state, these electrons are added to the level that will make them the most stable. It may be helpful to memorize the notation for these 2 elements, since they defy the rule. Cr = [Ar] 4 s 2 3 d 5 {\displaystyle 4s^{2}3d^{5}} {\displaystyle 4s^{2}3d^{5}} Cu = [Ar] 4 s 1 3 d 10 {\displaystyle 4s^{1}3d^{10}} {\displaystyle 4s^{1}3d^{10}}

















Comments
0 comment