views
Familiarizing Yourself With the Periodic Table
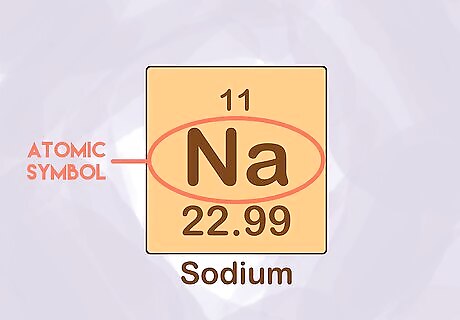
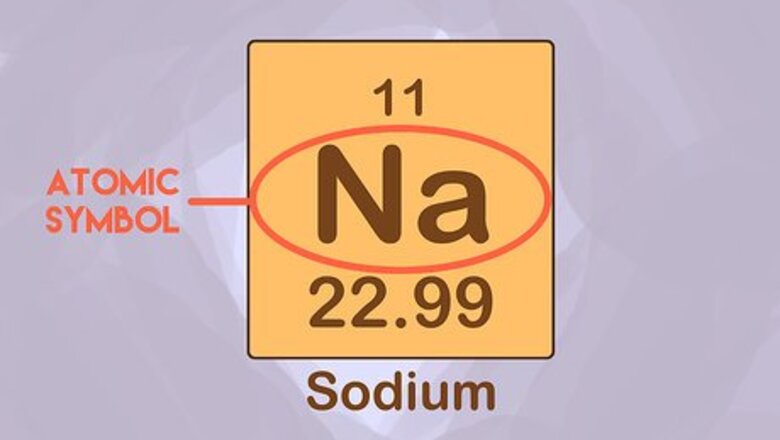
Memorize the atomic symbols. An atom is the smallest unit of an element that retains the properties of that element. Each atom has its own spot on the periodic table, and its own one or two letter symbol. For example, the symbol for hydrogen is “H” and sodium is “Na.” This often is a first assignment given to a chemistry class.
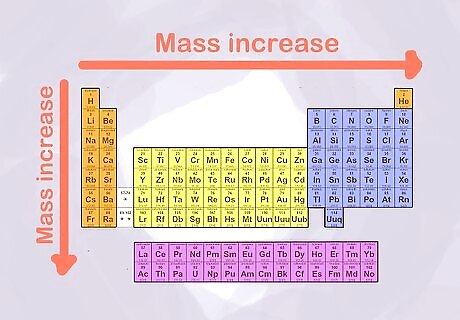
Know how mass changes in the table. One of the important characteristics of an atom is how much mass it has. This tells you how many particles are in the nucleus of the atom (the particles outside the nucleus are so small that they do not count in the mass). The periodic table is arranged so that mass increases as you go from left to right (across a row) and from top to bottom (down a column).
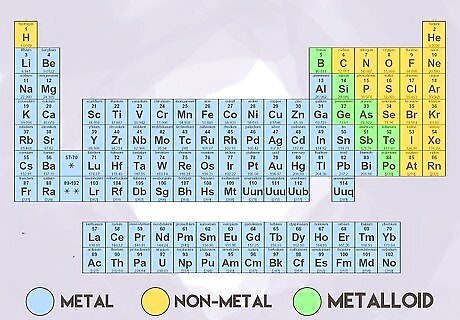
Understand the major types of elements. The periodic table is broken up into several different types of elements. The broadest categories are metals, metalloids, and nonmetals. The category that an atom belongs to can tell you about its physical properties and its reactivity.
Knowing the Composition of Atoms
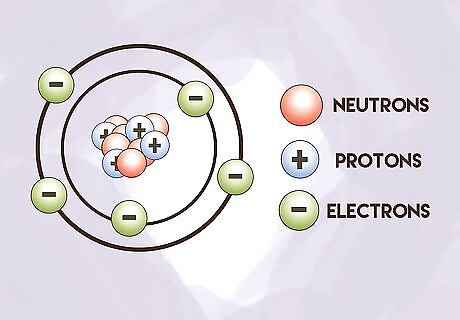
Know what subatomic particles make an atom. Three different subatomic particles make up an atom. Protons have a positive charge, neutrons lack charge, and electrons have a negative charge. The sum of protons and neutrons is equal to the mass of the atom (in atomic mass units). The mass of electrons is so little that they are not counted.
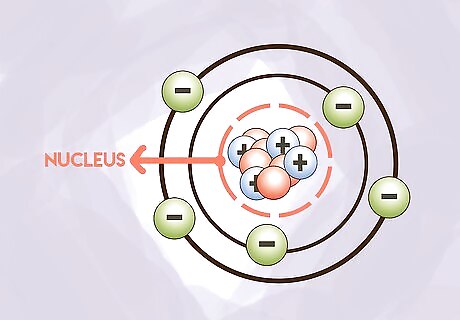
Be able to locate each particle. The nucleus is the center of the atom. This is where you will find protons and neutrons. Electrons orbit the nucleus in electron orbitals. Electrons do not typically enter the nucleus and protons and neutrons do not typically leave the nucleus.
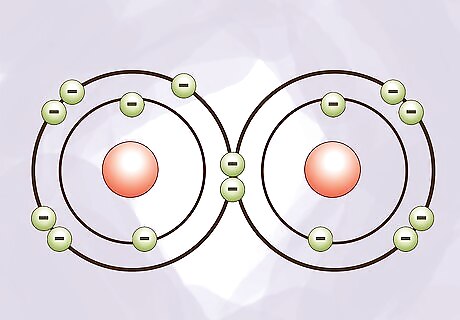
Consider what happens when atoms bond. When two or more atoms join together, it is called bonding. Protons and neutrons are not directly involved in bonding. Electrons are shared or transferred between two or more atoms in a bond and this forms a molecule.
Grasping Reactions and Bonding
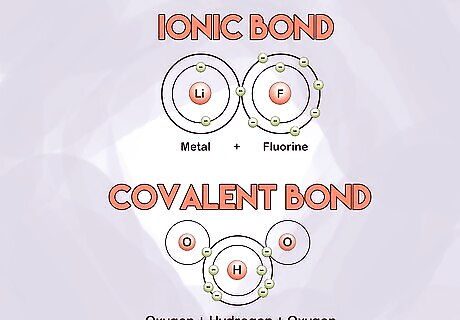
Learn the major types of bonds. Ionic bonds occur between metals and nonmetals. Covalent bonds occur between two nonmetals. In an ionic bond, electrons are more likely to be found at one end of a molecule than the other. This results in a negatively charged and positively charged end of the molecule. Covalent bonds share electrons more evenly.

Utilize chemical formulas. Chemical formulae provide information about what elements are present in a molecule or reaction, and in what proportions. For example, hydrochloric acid has a chemical formula of HCl and the chemical reaction describing burning methane is CH4 + 2O2 → 2H2O + CO2. The numbers before the chemical symbols are known as coefficients and tell you how many of that molecule or atom is present. The number in subscripts tell you how many of particular atom is present in the molecule.
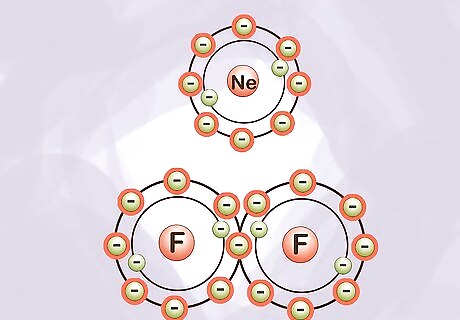
Consider stability as a reason for bonding. Atoms and molecules bond together to become more stable. That is to say that they try to attain the lowest possible energy state. To break a bond, you have to add enough energy to make the bond no longer favorable. For this reason, bonds are often hard to break.
Getting a Chemistry Education
Polish your math skills. To understand chemistry, you will need to have a working knowledge of algebra (at least) since you will be using it often. Understanding calculus, differential equations, and linear algebra is a huge plus in upper level chemistry courses, like physical chemistry. You will be required to take some of these courses to obtain your chemistry degree. You should also consider seeking a math minor (or even double major) to give yourself a leg up in your chemistry work.
Choose a branch of chemistry. Chemists are broken up into several different categories of study. For example, there are physical chemists, biochemists, analytical chemists, etc. Decide what kind of chemistry you like the most, and concentrate on that topic. For example, if you really like organic chemistry, take more organic chemistry courses than what's required for your degree. You can also pursue a master’s or doctorate to further narrow your scope and build your expertise in chemistry.
Do research in chemistry. Classwork is a good place to start learning the concepts of chemistry. Lab classes will help to teach you the protocol for conducting yourself in a laboratory and apply concepts you’ve learned in class. Ultimately though, if you wish to understand chemistry, you will have to do research on a topic in chemistry. While research is required for most chemistry degrees, it is usually a minimal amount. Start doing research as early as you can under the supervision of a professor in the field you like most (e.g. biochemistry).



















Comments
0 comment